All published articles of this journal are available on ScienceDirect.
Hospitalization Costs and Clinical Outcomes of Children with COVID-19: A Cross-Sectional Study from Iran
Abstract
Background
Age disparities in infection rates, clinical symptoms, and death rates during the COVID-19 pandemic were evident. However, there is insufficient evidence regarding the costs and outcomes of child cases and their associated factors.
Objective
This study aimed to investigate hospitalization costs and clinical characteristics/outcomes and their associated factors in Iranian children with COVID-19.
Methods
This retrospective descriptive-analytical study included children aged ≤18 years who were infected with COVID-19 and hospitalized for more than 24 hours. Hospitalization costs and death as outcome were the primary variables. Clinical data were obtained from a COVID-19 registry system in Iran, covering the years 2020-2021. Data analysis was performed using descriptive statistics and the generalized linear models (GLM) regression by employing STATA 15 software.
Results
A total of 698 children under 18 years old were included. Fever (75.2%), dry cough (43.0%), and vomiting (36.0%) were the most common symptoms. About 5.7% of the cases resulted in death. The mean hospitalization costs per patient were $2,820. Comorbidities and intensive care unit (ICU) admissions were factors associated with death, and age, ICU admissions, and comorbidities were factors associated with cost.
Conclusion
During epidemics, due to unknown clinical features and hidden symptoms, all age groups should be considered. Early diagnostic tests should be performed to reduce the spread of the virus, ensure timely treatment, and reduce medical costs after the appearance of initial symptoms.
1. INTRODUCTION
The 2030 Agenda for Sustainable Development envisioned ambitious hopes for the peace and prosperity of the world in the present and future by bringing together the leaders of countries around the world to focus on 17 key targets. Reducing avoidable and premature deaths attributable to communicable and non-communicable diseases was one of the Sustainable Development Goals (SDG) target in the health sector [1], which was significantly impacted by the COVID-19 pandemic. According to the Global Burden of Diseases (GBD) report, in 2019, more than 56 million people died due to various causes [2]. COVID-19 alone caused more than 6 million deaths between 2020 and 2023, significantly impacting the global burden of disease and death [3].
Even though the 2030 SDGs aim to reduce overall premature mortality, special attention has been given to reducing child and infant mortality, targeting reductions to 25 and 12 per 1000 live births of children aged under 5 and neonatal mortality, respectively, by 2030 [4, 5]. Projections made before the outbreak of COVID-19 indicated that these goals would not be achieved in a significant number of countries by 2030. The impacts of COVID-19 further darkened this outlook [6]. From the beginning of the COVID-19 pandemic, age disparities in infection rate, clinical symptoms, and death rate were evident [7-9]. Davies et al. estimated that individuals over 20 years of age were nearly twice as susceptible to infection compared to those under 20 [7]. According to Sasson, COVID-19 mortality increases exponentially with age [8]. COVID-19 infections in children were generally benign, although the cause of this tolerance is still unknown. It is also not definitively clear whether specific populations of children, including those with chronic diseases, have had similarly favorable outcomes [10].
In addition to the various effects that COVID-19 had on societies by increasing morbidity and mortality [11, 12] and reducing the quality of life (QALY) [13, 14], it also had numerous financial and economic consequences [15]. The financial loss imposed on the US hospital and health system due to COVID-19 was estimated at $50.7 billion per month [16]. According to Di Fuscoa's estimates, the median hospital costs for patients with COVID-19 in the United States were about $12,000 [17]. In Iran, several studies have been conducted to identify the clinical characteristics of patients with COVID-19 [18-20], estimating their costs [21], and economic burden as well [22, 23], but little evidence has been provided for the children. Therefore, the present study aimed to investigate the factors associated with the costs and outcomes of COVID-19 infection in Iran.
2. MATERIALS AND METHODS
2.1. Study Design and Population
This retrospective descriptive analytical study focused on the hospitalization costs and outcomes of COVID-19-infected children and the determination of the associated factors who were hospitalized for more than 24 hours in a specialized children's hospital in 2020-2021.
2.2. Clinical and Cost Data
Data on clinical characteristics and outcomes were extracted from the registry of patients with COVID-19 at Tehran University of Medical Sciences. The project was implemented at the beginning of the COVID-19 outbreak in selected hospitals, which were the main treatment centers for patients with COVID-19. Those who visited the centers were selected through random sampling.
The data obtained from the medical records were entered into a web-based system designed for disease registration. The medical records of about 20,000 hospitalized patients have been registered in the system. The records included demographic information, symptoms, time of onset of symptoms, history of previous hospitalizations and outpatient visits, comorbidities and important risk factors, the results of COVID-19 diagnostic tests, drug prescriptions, history of admission in critical care units, the need for mechanical ventilation, and finally, the condition at discharge time (survived or dead). One of the most important variables in the above list was comorbidities and key risk factors. These included a wide range of risk factors, such as obesity, as well as diseases, like asthma, cancer, neurological disorders, immunodeficiency, liver and kidney diseases, COPD, diabetes, and more. Given the length of this list, conducting subgroup analysis separately for all of the factors was challenging. Therefore, patients were categorized into three groups based on these variables: 1) without reported underlying condition, 2) with one comorbidity, and 3) with more than one comorbidity.
All therapeutic and diagnostic interventions for all COVID-19 patients studied were examined to estimate the hospitalization costs. The cost data were extracted from the Hospital Information System (HIS). Given that the present study was conducted retrospectively, the lack of access to reliable cost data due to recall bias has made it impossible to gather cost data before and after the hospitalization phase and estimate the total direct or indirect medical costs. Therefore, only the hospitalization costs of the patients have been presented. These included charges for laboratory tests, imaging, medication, hoteling, nursing care, visits, and consultations.
2.3. Data Analysis
Data from 698 children under 18 years old infected with COVID-19 were extracted and analyzed using STATA software version 15. In addition to providing descriptive metrics, such as the description of the age-gender distribution of patients, the frequency/prevalence of various disease symptoms, and outcomes, like ICU admissions and mortality, the mean and standard deviation of costs across different cost groups were calculated and two types of regression analyses were employed to determine the factors influencing mortality and leading to increased costs, as follows:
2.3.1. Multiple Logistic Regression
It was used to estimate the odds ratio of death influenced by various factors considered in the model and compare them.
2.3.2. Generalized Linear Regression Model (GLM)
It was used to investigate determinants of hospitalization cost of patients. Medical cost data are usually right-skewed, with variability increasing as the mean costs increase. The traditional model used to analyze and predict costs was multivariable ordinary least squares regression (OLS), applied with or without various transformations, including logarithmic transformations of the dependent variable (cost). The use of these transformed forms creates many problems in the interpretation and use of the results of such models. Therefore, in recent years, it has been suggested that for cost data, whose distribution is mainly skewed, generalized linear models (GLMs) should be used [24, 25]. Several recent studies on costs in the health sector have employed this approach [26, 27].
3. RESULTS
The clinical characteristics and hospitalization cost of 698 children under 18 years old with COVID-19 are presented as follows:
3.1. Demographic and Clinical Characteristics of Cases
About 55% of the studied cases were male, and 51% were ≤5 years old (Fig. 1). A total of 109 patients were admitted to the ICU, and about 5.7% of them died. The outcomes of the cases in the present study are summarized in Fig. (2).
Children infected with COVID-19 had a wide range of symptoms, the frequency and percentage of which are shown in Fig. (3). The main symptoms included fever (75.2%), dry cough (43.0%), vomiting (36.0%), dyspnea (26.8%), diarrhea (26.4%), anorexia (24.5%), and weakness and lethargy (24.4%) (Fig. 3).
Based on the results of multiple logistic regression analysis (Table 1) conducted to identify the factors associated with death in children with COVID-19, comorbidity and ICU admission significantly increased the likelihood of death. These variables were considered proxies of the severity of the disease. The odds ratio for children with more than one comorbidity was about 12 times greater than for children without comorbidity [OR=11.79, CI=95% (1.35–182.69, P-value<0.05)]. Additionally, as the severity of the child's illness increased, subsequently requiring prolonged critical care, the probability of death increased substantially.
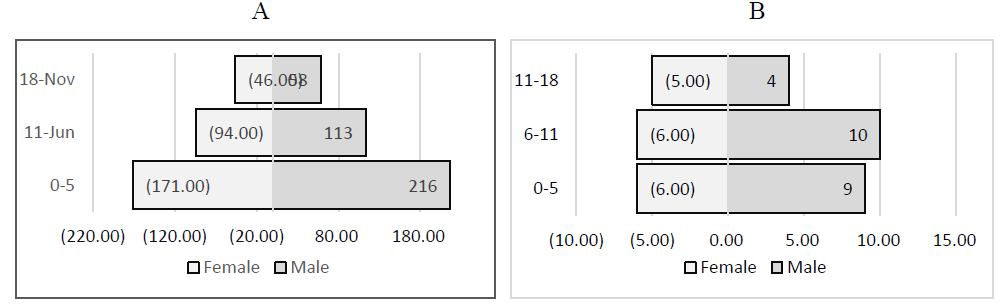
Age-gender distribution in the research sample. (A) Hospitalized patients; (B) patient death.
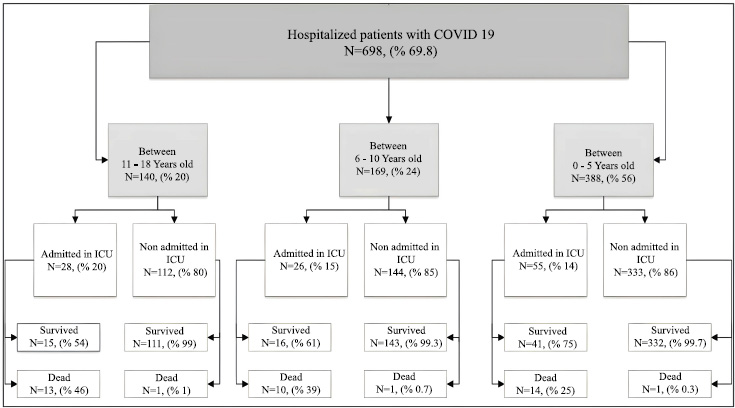
Outcomes of the cases in the present study.
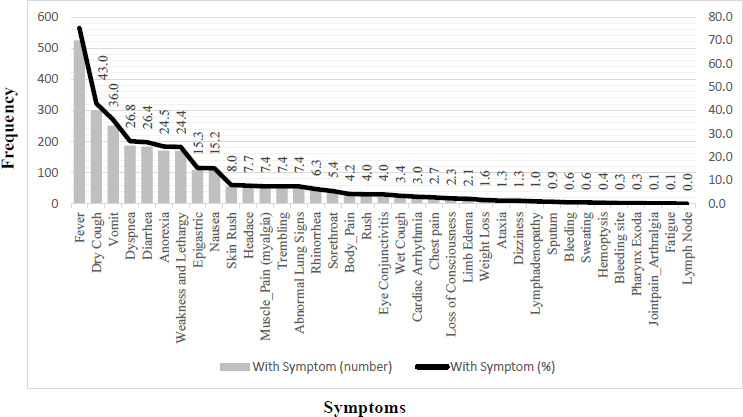
Frequency of symptoms in children hospitalized with COVID-19.
| Variable |
Survived | Dead | Odds Ratio | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Subgroups | Frequency | % | Frequency | % | OR | 95% Cl | |||
| Gender | Female | 290 | 94.46 | 17 | 5.54 | ref | |||
| Male | 351 | 93.85 | 23 | 6.15 | 1.72 | 0.71 | 4.18 | 0.227 | |
| Age group |
0-5 | 361 | 96.01 | 15 | 3.99 | ref | |||
| 6-10 | 155 | 93.37 | 11 | 6.63 | 1.78 | 0.65 | 4.89 | 0.260 | |
| 11-18 | 125 | 89.93 | 14 | 10.07 | 2.08 | 0.78 | 5.54 | 0.140 | |
| Hospital length of stay |
1-9 | 569 | 97.10 | 17 | 2.90 | ref | |||
| 10-20 | 52 | 82.54 | 11 | 17.46 | 0.80 | 0.29 | 2.22 | 0.678 | |
| >20 | 20 | 62.50 | 12 | 37.50 | 1.76 | 0.60 | 5.14 | 0.297 | |
| ICU admission | No | 572 | 99.48 | 3 | 0.52 | ||||
| Yes | 69 | 65.09 | 37 | 34.91 | 65.00 | 17.62 | 239.82 | 0.000 | |
| Comorbidity | No | 350 | 99.72 | 1 | 0.28 | ref | |||
| One | 195 | 87.05 | 29 | 12.95 | 18.43 | 2.33 | 145.59 | 0.006 | |
| More | 96 | 90.57 | 10 | 9.43 | 11.79 | 1.35 | 182.69 | 0.025 | |
| cons | 0.000 | 0.000 | 0.000 | 0.003 | |||||
| Total Cost | Lab | Imaging | Medicine | Hoteling and Nursing Care |
Visit and Consultation |
Other Services | |
|---|---|---|---|---|---|---|---|
| Mean | 2,820.63 | 245.26 | 41.93 | 955.26 | 1,009.29 | 212.87 | 316.10 |
| Median | 1,147.52 | 136.20 | 16.52 | 211.43 | 419.76 | 133.66 | 90.84 |
| Std. deviation | 5,580.91 | 396.58 | 78.53 | 2,667.26 | 1,823.04 | 249.31 | 1,003.53 |
| Service Groups |
Measure | Gender | Age Groups |
ICU Admission |
Comorbidity | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | 0-5 | 5-10 | 11-18 | No | Yes | No | 1 | >1 | ||
| Lab | Mean | 228.57 | 258.97 | 220.97 | 279.46 | 270.14 | 161.45 | 699.92 | 155.94 | 330.93 | 360.01 |
| St. dev. | 280.86 | 470.74 | 426.07 | 388.82 | 311.92 | 183.73 | 766.06 | 139.69 | 465.60 | 661.42 | |
| P-value | 0.904 | 0.001 | 0.000 | 0.000 | |||||||
| % of T (mean) | 13.34 | 13.42 | 14.09 | 13.53 | 11.32 | 14.43 | 7.75 | 15.22 | 11.54 | 11.22 | |
| % of T (S.D) | 8.09 | 8.22 | 8.12 | 8.32 | 7.72 | 8.30 | 4.01 | 8.24 | 7.37 | 8.09 | |
| P-value | 0.884 | 0.000 | 0.000 | 0.000 | |||||||
| Imaging | Mean | 42.97 | 41.08 | 36.07 | 46.12 | 52.79 | 24.03 | 139.05 | 24.35 | 56.01 | 70.38 |
| St. error | 80.23 | 77.20 | 66.64 | 77.55 | 104.37 | 39.97 | 141.16 | 48.51 | 84.06 | 121.69 | |
| P-value | 0.563 | 0.819 | 0.000 | 0.000 | |||||||
| % of T (mean) | 1.90 | 1.71 | 1.80 | 2.14 | 1.36 | 1.83 | 1.63 | 1.79 | 1.82 | 1.77 | |
| % of T (S.D) | 2.39 | 2.59 | 1.89 | 3.88 | 1.67 | 2.65 | 1.43 | 2.18 | 3.19 | 1.76 | |
| P-value | 0.435 | 0.012 | 0.150 | 0.559 | |||||||
| Medicine | Mean | 893.83 | 1,005.68 | 607.70 | 978.87 | 1,867.20 | 407.50 | 3,926.57 | 402.69 | 1,438.63 | 1,763.51 |
| St. error | 2,853.12 | 2,507.18 | 1,798.92 | 1,654.09 | 4,671.53 | 694.35 | 5,734.28 | 892.37 | 2,459.88 | 5,328.15 | |
| P-value | 0.667 | 0.000 | 0.000 | 0.000 | |||||||
| % of T (mean) | 24.69 | 25.37 | 21.17 | 27.95 | 32.14 | 23.66 | 32.64 | 23.71 | 27.89 | 23.54 | |
| % of T (S.D) | 13.86 | 14.98 | 10.64 | 15.48 | 18.36 | 14.39 | 12.56 | 14.00 | 14.99 | 14.22 | |
| P-value | 0.929 | 0.000 | 0.000 | 0.000 | |||||||
| Hoteling and nursing care | Mean | 981.44 | 1,032.15 | 937.29 | 957.80 | 1,265.55 | 539.26 | 3,559.02 | 520.75 | 1,416.35 | 1,766.81 |
| St. error | 1,723.12 | 1,903.12 | 1,837.56 | 1,466.77 | 2,131.91 | 834.18 | 3,153.81 | 901.78 | 2,017.95 | 2,904.07 | |
| P-value | 0.962 | 0.003 | 0.000 | 0.000 | |||||||
| % of T (mean) | 36.92 | 36.12 | 38.32 | 33.91 | 34.57 | 36.05 | 38.81 | 35.75 | 35.50 | 40.96 | |
| % of T (S.D) | 13.60 | 13.76 | 13.77 | 12.49 | 14.14 | 13.77 | 13.04 | 13.66 | 13.80 | 12.74 | |
| P-value | 0.421 | 0.000 | 0.049 | 0.001 | |||||||
| Visit and consultation | Mean | 202.65 | 221.26 | 190.48 | 224.96 | 259.01 | 153.62 | 534.29 | 146.07 | 276.53 | 299.53 |
| St. error | 217.60 | 272.60 | 218.36 | 257.45 | 306.22 | 137.67 | 418.81 | 140.70 | 291.03 | 354.29 | |
| P-value | 0.999 | 0.015 | 0.000 | 0.000 | |||||||
| % of T (mean) | 11.99 | 11.91 | 12.96 | 11.26 | 10.00 | 12.90 | 6.74 | 13.06 | 11.07 | 10.08 | |
| % of T (S.D) | 5.62 | 6.13 | 5.89 | 5.84 | 5.41 | 5.79 | 3.15 | 5.74 | 6.26 | 4.83 | |
| P-value | 0.344 | 0.000 | 0.000 | 0.000 | |||||||
| Other services | Mean | 261.46 | 360.95 | 265.47 | 345.63 | 417.76 | 134.53 | 1,301.03 | 108.99 | 466.05 | 685.03 |
| St. error | 622.70 | 1,230.20 | 1,033.93 | 830.59 | 1,101.91 | 244.74 | 2,243.82 | 141.44 | 949.62 | 2,052.17 | |
| P-value | 0.632 | 0.002 | 0.000 | 0.000 | |||||||
| % of T (mean) | 9.32 | 9.32 | 9.41 | 9.36 | 9.04 | 9.02 | 10.96 | 8.50 | 10.06 | 10.50 | |
| % of T (S.D) | 4.76 | 5.28 | 4.34 | 5.83 | 5.81 | 4.13 | 8.28 | 3.41 | 6.43 | 5.82 | |
| P-value | 0.439 | 0.012 | 0.782 | 0.007 | |||||||
| Variable | Subgroups | Mean | S.D | Coefficient | 95% CI | P-value | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Gender | Female | 373.32 | 232.47 | Ref. | |||
| Male | 392.00 | 272.73 | 20.66 | -4.37 | 45.70 | 0.106 | |
| Age groups | 0-5 | 343.94 | 190.18 | Ref. | |||
| 6-10 | 414.32 | 314.85 | 50.81 | 18.52 | 83.10 | 0.002 | |
| 11-18 | 454.08 | 306.71 | 43.89 | 8.72 | 79.05 | 0.014 | |
| ICU admission | No | 328.45 | 182.64 | Ref. | |||
| Yes | 682.62 | 364.71 | 205.01 | 141.98 | 268.04 | P<0.001 | |
| Comorbidity | No | 319.25 | 125.00 | Ref. | |||
| 1 | 459.93 | 298.46 | 27.97 | -2.01 | 57.97 | 0.068 | |
| >1 | 435.26 | 389.95 | 27.27 | -14.55 | 69.11 | 0.201 | |
| Cons | 211.57 | 111.54 | 311.60 | 0.000 | |||
3.2. Hospitalization Costs
The descriptive statistics with respect to costs are presented in Table 2. The average hospitalization cost per patient was $2,820, with a median of $1,147. Among the cost components, hotel and nursing services ($1,009) and medications ($955) accounted for the largest proportion of total expenses.
The costs across different subgroups of patients' medical bills, along with the results of the Mann-Whitney and Kruskal-Wallis tests conducted to assess the statistical significance of cost differences among these subgroups, are presented in Table 3.
Expenditures on diagnostic and treatment services were higher in the 5–10 and 11–18 age groups compared to children under 5 years old. However, the difference was not statistically significant for imaging services (P > 0.05). Additionally, no significant cost difference was observed between male and female patients. Patients admitted to the ICU and those with comorbidities incurred higher costs than their respective comparison groups.
Based on the regression analysis using the generalized linear model (GLM) (Table 4), age, ICU admission, and comorbidities had a significant impact on costs. The effect of gender on costs could not be ruled out at a significance level of 0.1 (95% CI: 2.61–49.01, P < 0.1).
4. DISCUSSION
Patients with COVID-19 have been reported to exhibit various clinical characteristics and symptoms in some studies [28-31]. However, other studies have specifically investigated the clinical characteristics of children with this infection [29, 32-34]. The present study focused on the clinical characteristics, hospitalization costs, and associated factors in 698 children with COVID-19 in a children's hospital in Iran during the pandemic. Based on our findings, fever (75.2%), dry cough (43.0%), vomiting (36.0%), dyspnea (26.8%), diarrhea (26.4%), anorexia (24.5%), and weakness and lethargy (24.4%) were the most important symptoms among Iranian children with COVID-19 being under 18 years old. Several studies have documented the clinical presentation of COVID-19 in pediatric populations. As highlighted in the reviews by She et al. [35] and Tsai et al. [36], common symptoms in children with COVID-19 include cough, fever, vomiting, headache, and abdominal pain. Castagnoli et al., in a systematic review, concluded that children generally experience mild or asymptomatic infections, with the most frequently reported symptoms being dry cough, fever, and fatigue. Notably, no fatalities have been reported in this pediatric cohort [37]. Duarte-Salles et al., based on an international network cohort comprised of 9,769 hospitalized cases and 242,158 non-hospitalized cases, demonstrated a higher prevalence of comorbidities, such as cardiovascular disease, neurodevelopmental disorders, and cancer, among hospitalized patients compared to non-hospitalized cases. Furthermore, the mortality rate among children with COVID-19 has been found to be negligible [38].
Our study found that 5.7% of cases resulted in mortality, with all fatal cases presenting severe symptoms. This underscores the importance of comparing mortality rates between pediatric and adult populations, as such comparisons could offer critical clinical insights. Tsai et al. and Murillo-Zamora et al. [36, 39] have indicated a significantly lower mortality rate in children with COVID-19 compared to adults. This difference may be attributed to the typically mild or asymptomatic course of the disease in pediatric cases and its lower prevalence among children, as highlighted by Miri et al. [40]. Various estimates of the case fatality rate of COVID-19 in children have been presented globally; for example, through a population-based estimation in Korea, it was reported as 0.85 per 100,000 COVID-19 cases [41]. According to a UNICEF published report in 2023, children represented approximately 0.4% of the total number of COVID-19-related deaths worldwide [42]; in a study from India, among 475 hospitalized children with COVID-19, the all-cause mortality rate was 9.9%, but the disease-specific mortality attributed directly to COVID-19 was only 1.9% [42]. Considering the low infection rate of children with COVID-19 and their mortality rate across the total population, hospital data has reported higher mortality rates because the target population has consisted of individuals with severe to critical stages. For instance, a study based on data from 303,887 hospitalized COVID-19 patients during the first 14 months of the pandemic in Iran indicated 14,111 cases to be under 20 years old [4.64%], and of these hospitalized cases, 4.75% resulted in fatalities (703 cases) [43]. A study, aimed at estimating the burden of moderate to critical COVID-19 cases in Iran during 2020 and 2021, reported a total of 590 and 851 deaths, respectively, among individuals under 20 years old. This resulted in a cumulative mortality count of 1,441 over the two-year period. Given that the total number of hospital admissions in this age group during the same timeframe was 43,628, the hospital mortality rate was estimated at 3.3% [44]. Therefore, considering this issue, a mortality rate of 5.7% in a specialized children's hospital seems reasonable, given the evidence from other studies mentioned.
Comorbidities were identified as a significant factor associated with mortality. Our study found that the risk of death among children with comorbidities was approximately 12 times higher compared to those without underlying conditions. However, a detailed subgroup analysis could not be conducted due to the wide range of comorbidities present. Similarly, W. McCormick et al. assessed the clinical characteristics of approximately 110 COVID-19-related deaths among individuals under 21 years old in the United States in 2020, indicating 86% of cases to have at least one comorbidity. The most prevalent comorbidities included obesity, asthma, and developmental disorders [45]. According to Hann Ng’s systematic review and meta-analysis, which analyzed data from 2019 to 2020, the comorbidities most strongly associated with mortality in COVID-19 cases across all age groups included diabetes, hypertension, chronic kidney disease, and cancer. While obesity had the highest prevalence among comorbid conditions, it was not directly linked to an increased risk of death [46]. Yang et al.'s systematic review identified hypertension, respiratory system diseases, and cardiovascular disease as key comorbidities associated with increased disease severity in COVID-19 cases [47].
In the present case study, approximately 17% of cases required admission to the intensive care unit. Hospitalization due to COVID-19 is necessary for patients exhibiting severe symptoms, while ICU admission is typically reserved for the most critical cases. Although cultural and medical practices vary globally, reports from most healthcare centers indicate that approximately 25% of hospitalized patients require intensive care [48]. Bhuiyan et al.'s systematic review indicated that approximately 7% of pediatric COVID-19 cases required admission to the ICU [49]. Zajic et al., in their analysis of hospital outcomes among COVID-19 patients in Austria, reported that 12.3% of the 68,193 patients included in the study required admission to the ICU [50]. ICU admission rates also vary by age group. For instance, according to reports from the Centers for Disease Control and Prevention (CDC), 61.3% of ICU admissions have been found to be among adults aged 65 years and older [50]. A study conducted by Damiri et al. in Iran reported ICU admission rates of 23.05% among COVID-19 patients under 50 years old and 16.46% among those over 50 years old [44]. Due to variations in study settings and target populations, it was not possible to definitively determine whether the ICU admission rate observed in the present study, which focused on children, was relatively high or low. However, the reported rate appeared to be within a reasonable range.
According to the present study, the average hospitalization cost per patient was $2,820. The highest expenses were associated with hoteling and nursing care, accounting for 33% of the total costs, followed by medication costs, which comprised 35% of the total hospital expenses. Given the significant differences between countries in healthcare reimbursement structures, purchasing power parity, and other cost-determining factors, directly comparing the absolute costs of COVID-19 treatment estimated in this study with those reported in other countries may not be appropriate. However, numerous studies on this subject have been conducted in Iran in recent years. For example, The hospitalization costs per patient have been reported to be different in several studies, including $3755 [23], $2634.79 [22], $1434 [51], and $2,461 [52], respectively. Various studies have identified multiple factors influencing the cost of COVID-19 treatment in patients. For instance, comorbidity, age, severity of the disease, ICU admission, use of intranasal oxygen, and length of stay have been reported in a study by Kaso et al. [53].
It is important to note that ICU admission has been found to be one of the factors associated with higher hospitalization costs. This can be attributed to the increased use of medicines and longer length of stay due to the severity of the disease. The study by Fusco M et al., conducted from April 1 to October 31, 2020, reported ICU admission and IMV usage as significant factors associated with hospital costs and charges, with approximately 21.9% of patients being admitted to the ICU [17]. Based on a systematic review by Gholipour et al., with respect to costs of inpatient care for COVID-19 cases, the ICU incurred the highest hospitalization costs per patient. The highest and the lowest costs were 100,789 USD in Germany and 5,436.77 USD in Romania, respectively. Treatment costs (42.23%) and hospital bed day costs (39.07%) constituted the highest percentages of direct medical costs in the special care and inpatient departments, respectively [54]. On average, the cost per patient for ICU and non-ICU care in the Kingdom of Saudi Arabia was 79,418.30 and 42,704.49, respectively, between March 1 and May 29, 2020 [55]. According to the study by Ohsfeldt et al. involving 247,590 cases of COVID-19, the highest costs were associated with patients requiring mechanical ventilation from April 1 to December 31, 2020, in the USA. The median ICU and hospital costs were US $13,443 and $11,267, respectively. The daily costs were US $1772, and $2902, respectively [56].
Ohsfeldt et al. associated factors, such as age, race, comorbidities (e.g., obesity), treatment with oxygenation, and early discharge, with higher medical costs and increased mortality [56]. In a costing study by Oliveria et al., among 1084 COVID-19 cases in Brasilia from March and September 2020, obesity, age, and sex were identified as cost predictors from the hospital and the healthcare system perspective, respectively [57]. Age and death were previously identified as factors associated with costs. However, based on the present study results, ICU admission and comorbidities were the primary factors. Cases admitted to the ICU incurred higher laboratory test costs ($699.92), as did cases with comorbidities ($360.01) and those between 5-10 years old ($279.46). Medicine costs were highest in cases between 11-18 years old ($1,867.20). The costs of medicine for ICU admissions and cases with comorbidities were $3,926.57 and $1,763.51, respectively.
All of the studies have highlighted the importance of screening to reduce costs and improve the quality of life for individuals and society. The COVID-19 pandemic could be mitigated by screening strategies, and educational programs could increase the efficiency of screening, as confirmed by Ravindra et al. in their systematic review study. According to their results, asymptomatic infection is a significant factor in the transmission of COVID-19 within communities. While asymptomatic cases have not been as apparent in children, constant screening, monitoring, and mitigation strategies for children, especially those under 18 years old, are essential to prevent the spread of the virus [58]. Mohammad Mosadeghrad et al. recommended primary health care (PHC) strategies based on a scoping review on COVID-19. They emphasized the importance of adaptable, well-resourced PHC systems capable of maintaining continuity and effectively managing health services. These strategies have been introduced as essential measures during emergencies, such as the COVID-19 pandemic [59].
Although our study is the first in Iran to specifically examine the clinical and hospitalization costs of children with COVID-19 using a reliable data source from a supervised disease registry system, several limitations should be acknowledged. Due to the high number of risk factors and comorbidities, conducting a subgroup analysis in this regard was not feasible. Additionally, the retrospective nature of the study due to recall bias considerations and other data accessibility difficulties posed challenges in collecting comprehensive and reliable data on the additional financial burden imposed on families before and after hospitalization. Although this study explored the relationship between patient mortality and varying degrees of comorbidities, the current limitations in health information systems make it challenging to determine whether deaths were directly attributable to COVID-19 or if the virus indirectly contributed to mortality by exacerbating pre-existing conditions. Future research efforts should focus on designing efficient and resilient systems that equitably address the needs of different societal groups to enhance preparedness for crises, such as pandemics. A key element in this design must involve the development of data infrastructure for recording and tracking disease symptoms and monitoring disparities in health outcomes across various demographic groups, healthcare providers, and geographic regions. Identifying and analyzing these disparities, along with the integration of care programs, can significantly enhance the safety, effectiveness, and efficiency of healthcare systems during crises.
CONCLUSION
Comorbidity and ICU admission were significant factors associated with mortality and costs in children with COVID-19. Children aged 5-10 years and 11-18 years incurred higher expenses for diagnostic and treatment services compared to those under 5 years old. Additionally, ICU admissions resulted in higher costs than non-ICU admissions, and patients with comorbidities had higher costs across all sub-groups. During epidemics, it is crucial to consider all age groups due to the unknown clinical features and hidden symptoms. Early diagnostic tests should be performed to reduce the spread of the virus, ensure timely treatment, and minimize medical costs after the appearance of initial symptoms.
AUTHORS’ CONTRIBUTIONS
The authors confirm their contribution to the paper as follows: study conception and design: M.R., S.N., A.G., S.D., A.R.Y.; data collection: M.G., S.R., E.S.; conceptualization: E.H., Z.M. All authors have reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| CDC | = Centers for Disease Control and Prevention |
| GLM | = Generalized Linear Regression Model |
| GBD | = Global Burden of Diseases |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the ethics committee of Tehran University of Medical Sciences, Iran, with ID number IR.TUMS.MEDICINE.REC.1400.865.
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee, and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
A written informed consent was obtained from all the study participants/guardians.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
ACKNOWLEDGEMENTS
The authors would like to express their gratitude to the team members of the Tehran University of Medical Sciences for their invaluable assistance in gathering the data for this study.


